Home / PINNACLE® I.N.
-
-
The only two-dose modified-live vaccine developed to help prevent strangles caused byStreptococcus equi.
-
Intranasal administration provides a “more natural” immune response, stimulating innate and mucosal immunity at the site of natural infection.1
-
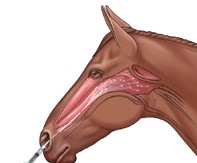
Pinnacle I.N. utilizes a specially designed cannula that helps deliver the vaccine to the pharyngeal (throat) area.
-
-
DOSE: Aseptically rehydrate with the entire contents of the accompanying sterile diluent. Instill the entire rehydrated vaccine into one nostril using a syringe with applicator tip. Administer a second dose 2 to 3 weeks later. Annual revaccination is recommended. FOR INTRANASAL USE ONLY. DO NOT ADMINISTER BY ANY ROUTE OTHER THAN INTRANASAL.
-
In a Pinnacle Field Safety Study2, 99.14% of vaccinates did not have a vaccine related reaction.
-
582 Vaccinated Horses—vaccinated twice 2–3 weeks apart.
-
110 Control Horses—co-mingled with vaccinates.
-
5/582 of first-time vaccinates had mild local or systemic reaction.
-
0/582 of second-time vaccinates had local or systemic reactions.
-
No severe local or systemic reactions.
-
Horses were monitored for fever, lethargy, anorexia and nasal discharge— 30 minutes, 1 week and 2 weeks post-vaccination.
0.43% vaccine-related incidence rate.
-
-
- C.R. Sweeney, J.F. Timoney, J.R. Newton, and M.T. Hines: Streptococcus equi Infections in Horses: Guidelines for Treatment, Control, and Prevention of Strangles. J Vet Intern Med 2005; 19:123–134.
- Data on file, Study Report No. 22741, Zoet Inc.is
-
This product contains live bacteria and is designed for intranasal use only. Disinfect hands and equipment after use. Contamination of the user’s hands or equipment with reconstituted vaccine could lead to infections if proper disinfection practices are not followed prior to procedures that require asepsis. Injection equipment used to reconstitute or administer Pinnacle I.N. should not be reused, and should be disposed of appropriately. In case of anaphylactoid reaction, administer epinephrine. After administration a small number of horses may experience non-contagious transitory upper respiratory signs including nasal discharge and lymphadenectasis. Purpura hemorrhagica may be seen in hypersensitive individuals following exposure to streptococcal proteins. Store in the dark at 2° to 7°C (35° to 45°F). AVOID FREEZING. Shake well after rehydration. Do not vaccinate within 30 days before slaughter. Use entire contents when first opened. Burn container and all unused contents

- Argentina
- Australia
- Austria
- Belgium
- Bolivia
- Brazil
- Bulgaria
- Canada
- Chile
- China
- Colombia
- Costa Rica
- Croatia
- Czech Republic
- Denmark
- Ecuador
- Egypt
- Estonia
- Ethiopia
- Finland
- France
- Germany
- Ghana
- Greece
- Hungary
- India
- Indonesia
- Ireland
- Israel
- Italy
- Japan
- Kenya
- Latvia
- Liberia
- Lithuania
- Malawi
- Malaysia
- Mauritius
- Mexico
- Morocco
- Mozambique
- Netherlands
- New Zealand
- Nigeria
- Paraguay
- Peru
- Philippines
- Poland
- Portugal
- Romania
- Russia
- Serbia
- Singapore
- Slovakia
- Slovenia
- South Africa
- South Korea
- Spain
- Switzerland
- Taiwan
- Tanzania
- Thailand
- Turkey
- Uganda
- Ukraine
- United Kingdom
- United States
- Uruguay
- Vietnam
- Zambia
- Zimbabwe
You are leaving the country website to access another site in the group.
Regulatory constraints and medical practices vary from country to country. Consequently, the information provided on the site in which you enter may not be suitable for use in your country.